Microscopic Anatomy
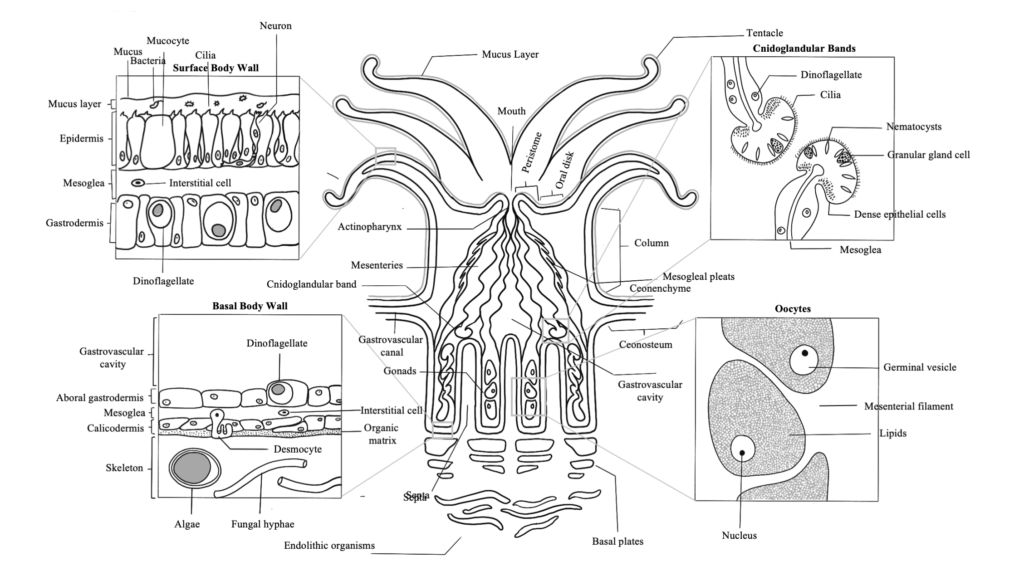
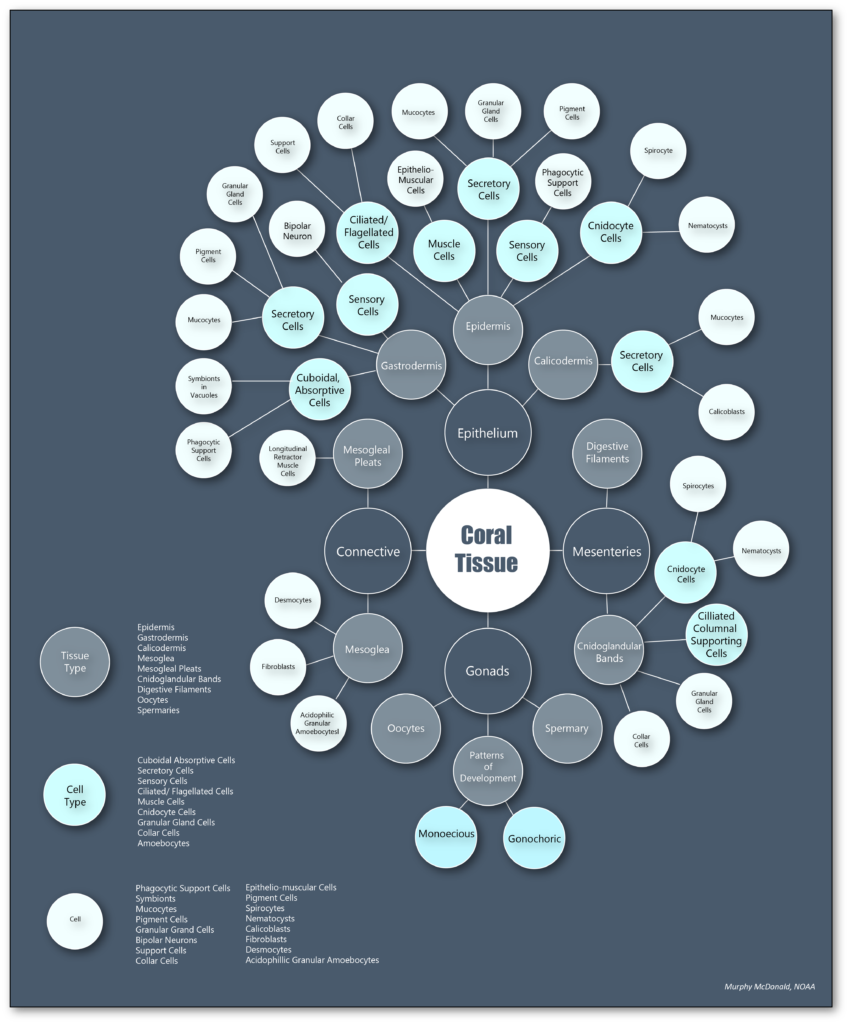
The fundamental structure of a coral polyp is a cylindrical sac lined composed of two layers of epithelia: the surface body wall, which forms the outermost barrier between the organism and its surroundings, and the basal body wall, which anchors the polyp to its skeleton. Water and ingested materials enter the polyp through the mouth at the distal (uppermost) region of the polyp; water and waste products are expelled by the polyp through the same opening. Respiration and excretion occur by direct exchange of molecules through the two layers of epithelia.
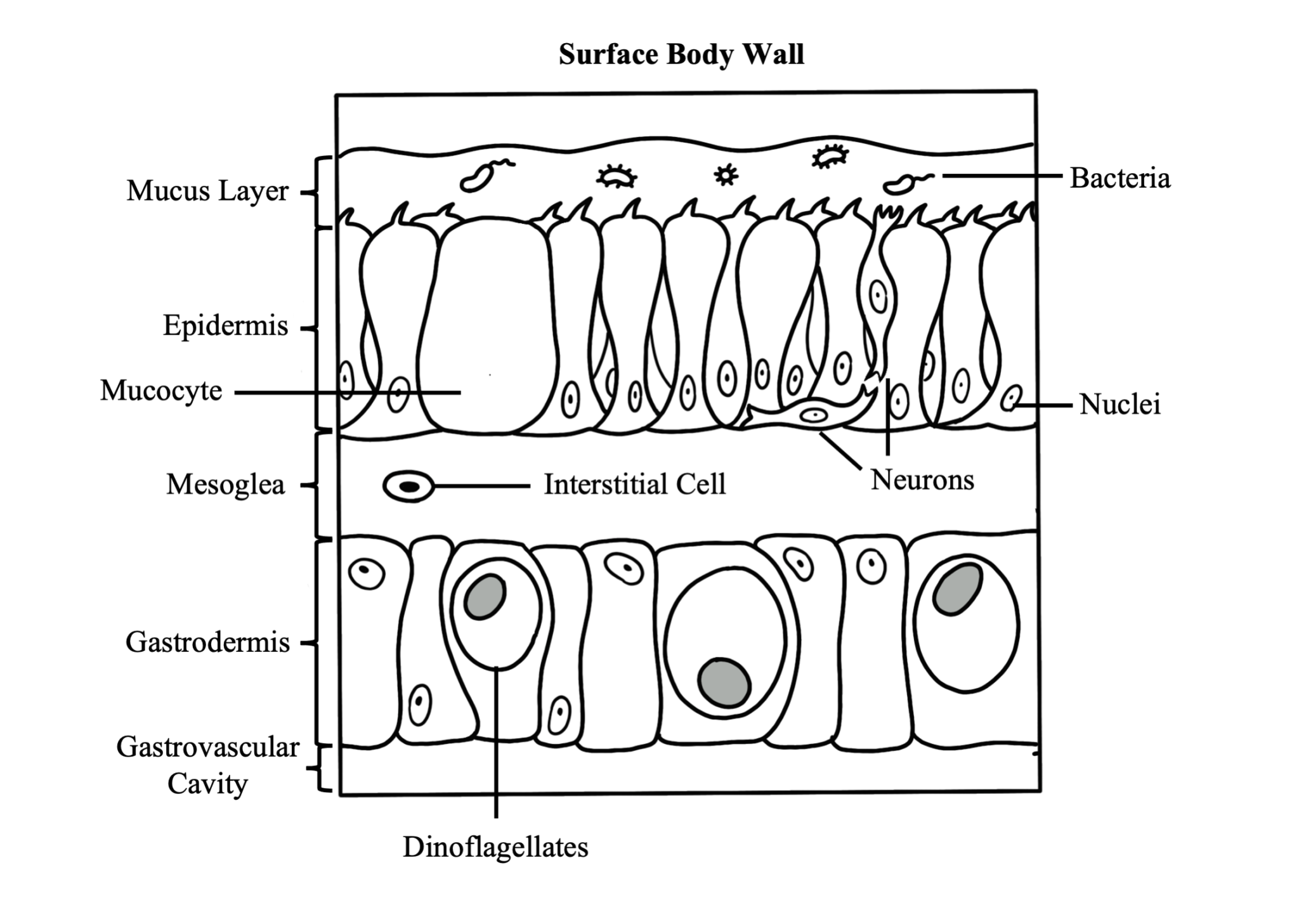
The surface body wall is the epithelial tissue that forms the outermost barrier of the organism and is responsible for producing the protective film of mucus that envelops coral colonies. The surface body wall consists of three tissue layers: the outermost layer known as the epidermis, the innermost layer known as the gastrodermis, and a layer of connective tissue between them known as the mesoglea.
The mesoglea supports the two epithelia and the epitheliomuscular cells of retractor muscles. This layer contains fibroblasts that secrete collagen and ground substance, and amoebocytes that serve as the principal cells of the coral’s immune system
The epidermis is a layer comprised of epithelium responsible for secreting the mucus layer that encompasses the organism and aids in protection, sediment removal, and feeding. Specialized cells in the epidermis are ciliated columnar support cells, mucocytes (secrete mucus), sensory bipolar neurons that connect to the subepidermal nerve net, cnidocytes (produce stinging cells called nematocysts or spirocytes), pigment cells, epitheliomuscular cells, and amoebocytes.
The gastrodermis lines the interior of the polyp and serves as its digestive system. Phagocytotic supporting cells contain symbiotic algal cells in vacuoles, which undergo photosynthesis and exchange nutrients and waste molecules with the coral host cells. Other cell types common throughout the gastrodermis are mucocytes, cnidocytes, granular gland cells, pigmented cells, epitheliomuscular cells, and scattered amoebocytes.
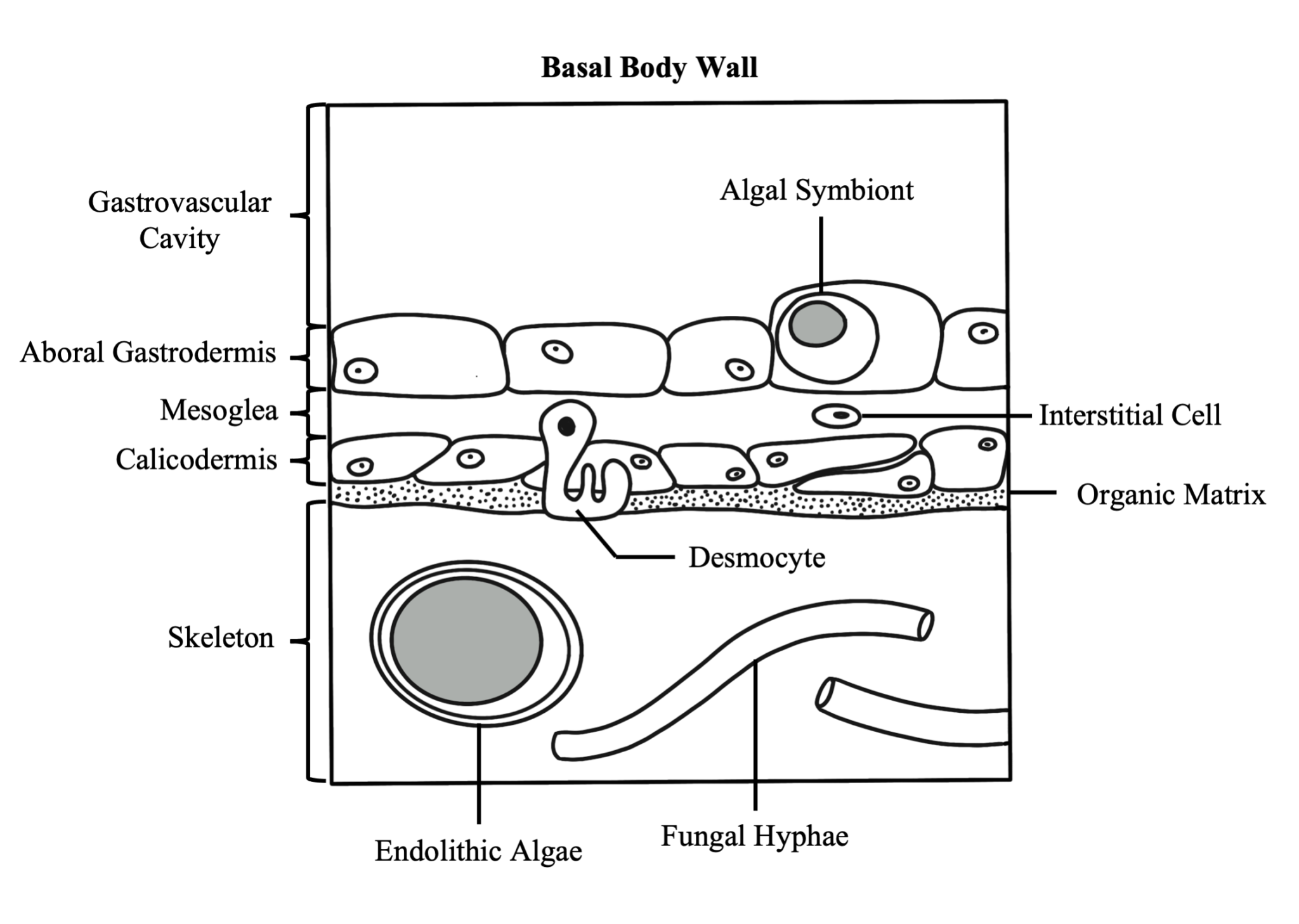
The basal body wall is likewise comprised of three tissue layers: the gastrodermis and mesoglea, which are both functionally similar to those layers in the surface body wall, and the calicodermis, which is the tissue layer responsible for creating the coral’s skeleton. Skeleton is formed outside of cells called calicoblasts, which secrete a soluble organic matrix that facilitates calcium carbonate crystal formation. The basal body wall also contains specialized epithelial cells called desmocytes, which interdigitate with the mesoglea and use specialized fibers to anchor the coral polyp to its skeleton.
Learn More about the Essentials of Coral Biology:
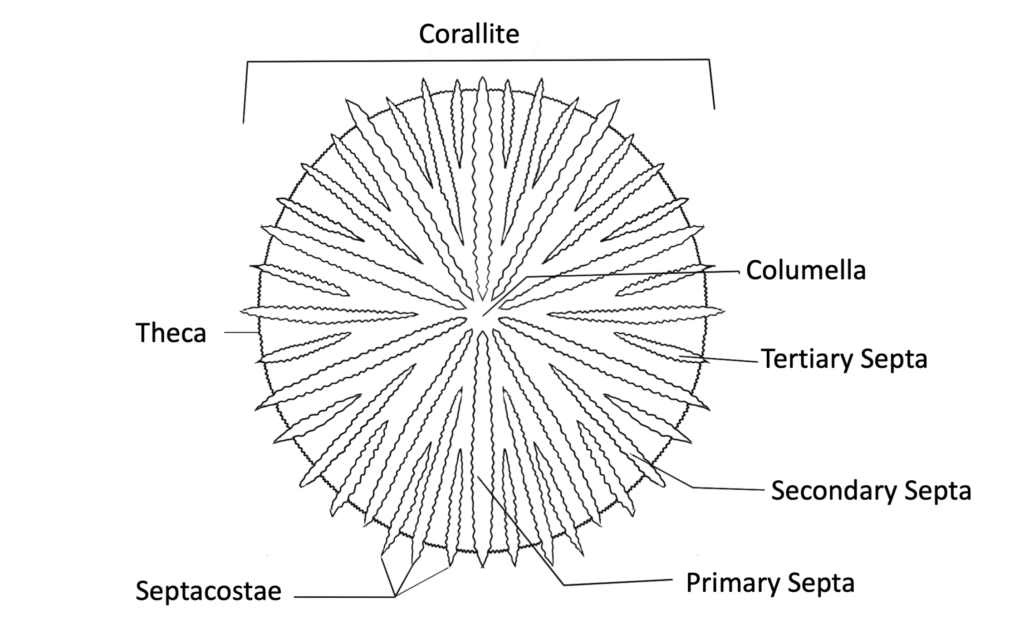
Skeletal Anatomy
Learn about all of the components of the coral skeleton and the different morphologies that it can display
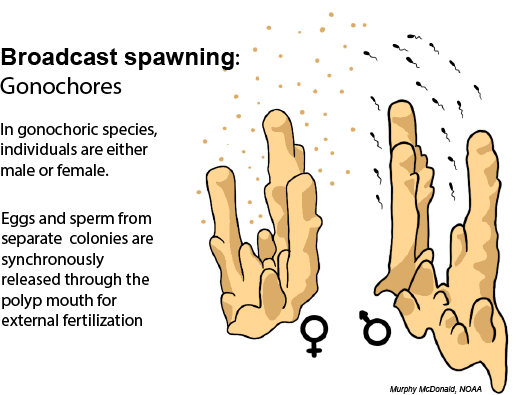
Coral Reproduction
Learn about how corals use different reproductive modes to overcome their immobility
Publications Relevant to Coral Anatomy:
Agassiz, L. (1849) On the structure of coral animals. Proceedings of the American Association for the Advancement of Sciences 2, 68-77.
Brown, B. E., & Bythell, J. C. (2005). Perspectives on mucus secretion in reef corals. In Marine Ecology Progress Series (Vol. 296). https://doi.org/10.3354/meps296291
Burton, P. M. (2008). Insights from diploblasts; the evolution of mesoderm and muscle. In Journal of Experimental Zoology Part B: Molecular and Developmental Evolution (Vol. 310, Issue 1). https://doi.org/10.1002/jez.b.21150
Cairns, S. D. (1991). A revision of the Ahermatypic Scleractinia of the Galapagos and Cocos Islands. Smithsonian Contributions to Zoology, 504. https://doi.org/10.5479/si.00810282.504
Chornesky, E., and Peters, E. (1987) Sexual Reproduction and colony growth in the reef coral Porites astreoides. Biological Bulletin 172, 161-177.
Epp, L., Smid, I., & Tardent, P. (1986). Synthesis of the mesoglea by ectoderm and endoderm in reassembled hydra. Journal of Morphology, 189(3). https://doi.org/10.1002/jmor.1051890306
Fautin, D., and Mariscal, R. (1991) Cnidaria: Anthozoa, in Microscopic Anatomy of Invertebrates: Placozoa, Porifera, Cnidaria, and Ctenophora (eds F. harrison and J. Westfall), Vol. 2. Wiley-Liss, New York, NY, pp. 267-358
Galloway, S., Work, T., Bochsler, V., et al. (2007) Coral Disease and Health Workshop: Coral Histopathology II. NOAA Technical Memorandum NOS NCCOS 56 and CRCP 4. National Oceanic and Atmospheric Administration, Silver Spring, MD.
Gateño, D., & Rinkevich, B. (2003). Coral polyp budding is probably promoted by a canalized ratio of two morphometric fields. Marine Biology, 142(5). https://doi.org/10.1007/s00227-003-1009-8
Gladfelter, E. H. (1932) Circulation of fluids in the gastrovascular system of the reef coral Acropora cervicornis. The Biological Bulletin, 165(3). https://doi.org/10.2307/1541469
Goldberg, W. M. (2002). Feeding behavior, epidermal structure and mucus cytochemistry of the scleractinian Mycetophyllia reesi, a coral without tentacles. Tissue and Cell, 34(4). https://doi.org/10.1016/S0040-8166(02)00009-5
Goldberg, W. M. (2002). Gastrodermal structure and feeding responses in the scleractinian Mycetophyllia reesi, a coral with novel digestive filaments. Tissue and Cell, 34(4). https://doi.org/10.1016/S0040-8166(02)00008-3
Goreau, T. F. (1956) A study of the Biology and Histochemistry of Corals. Ph. D. thesis, Yale University.
Grimmelikhuijzen, C. J., & Westfall, J. A. (1995). The nervous systems of cnidarians. In EXS (Vol. 72). https://doi.org/10.1007/978-3-0348-9219-3_2
Lang, J. (1984) Whatever works: The variabie importance of skeletal and of non-skeletal shcaracters in scleractinian taxonomy. Paleontographica Americana 54, 18-44.
Lang, J. C., & Chornesky, E. (1990). Competition between scleractinian reef corals – a review of mechanisms and effects. In Ecosystems of the world. Volume 25: Coral reefs (Vol. 25).
Lesser, M. P., Mazel, C. H., Gorbunov, M. Y., & Falkowski, P. G. (2004). Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science, 305(5686). https://doi.org/10.1126/science.1099128
Marshall, A. T., & Wright, O. P. (1993). Confocal laser scanning light microscopy of the extra-thecal epithelia of undecalcified scleractinian corals. Cell & Tissue Research, 272(3). https://doi.org/10.1007/BF00318560
Martindale, M. Q., Pang, K., & Finnerty, J. R. (2004). Investigating the origins of triplosblasty: “Mesodermal” gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development, 131(10). https://doi.org/10.1242/dev.01119
Mullen, K., Peters, E., and harvell, C. (2004) Coral resistance to disease, in Coral Health and Disease (eds E. Rosenberg and Y. Loya). Springer-Verlag, Berlin, pp. 377-399.
Muscatine, L., Ferrier-Pagès, C., Blackburn, A., Gates, R. D., Baghdasarian, G., & Allemand, D. (1998). Cell-specific density of symbiotic dinoflagellates in tropical anthozoans. Coral Reefs, 17(4). https://doi.org/10.1007/s003380050133
Muscatine, L., Tambutte, E., & Allemand, D. (1997). Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs, 16(4). https://doi.org/10.1007/s003380050075
Nothdurft, L. D., & Webb, G. E. (2009). Clypeotheca, a new skeletal structure in scleractinian corals: A potential stress indicator. Coral Reefs, 28(1). https://doi.org/10.1007/s00338-008-0439-7
Östman, C. (2000). A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Scientia Marina, 64(SUPPLEMENT 1). https://doi.org/10.3989/scimar.2000.64s131
Palmer, C. v., Bythell, J. C., & Willis, B. L. (2010). Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals. The FASEB Journal, 24(6). https://doi.org/10.1096/fj.09-152447
Palmer, C. v., Modi, C. K., & Mydlarz, L. D. (2009). Coral fluorescent proteins as antioxidants. PLoS ONE, 4(10). https://doi.org/10.1371/journal.pone.0007298
Palmer, C. v., Mydlarz, L. D., & Willis, B. L. (2008). Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proceedings of the Royal Society B: Biological Sciences, 275(1652). https://doi.org/10.1098/rspb.2008.0335
Peters, E., (1984) A Survey of the Normal and Pathological Histology of Scleractinian Corals with Emphasis on the Effects of Sedimentation Stress. Ph. D. dissertation, University of Rhode Island.
Peters, E., Price, K., & J. Borsay Horowitz, D. (2005). Histological preparation of invertebrates for evaluating contaminant effects. In Techniques in Aquatic Toxicology, Volume 2. https://doi.org/10.1201/9780203501597.ch36
Petes, L. E., Harvell, C. D., Peters, E. C., Webb, M. A. H., & Mullen, K. M. (2003). Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Marine Ecology Progress Series, 264. https://doi.org/10.3354/meps264167
Puverel, S., Tambutté, E., Zoccola, D., Domart-Coulon, I., Bouchot, A., Lotto, S., Allemand, D., & Tambutté, S. (2005). Antibodies against the organic matrix in scleractinians: A new tool to study coral biomineralization. Coral Reefs, 24(1). https://doi.org/10.1007/s00338-004-0456-0
Seipel, K., & Schmid, V. (2006). Mesodermal anatomies in cnidarian polyps and medusae. In International Journal of Developmental Biology (Vol. 50, Issue 7). https://doi.org/10.1387/ijdb.062150ks
Sharp, K. H., Ritchie, K. B., Schupp, P. J., Ritson-Williams, R., & Paul, V. J. (2010). Bacterial acquisition in juveniles of several broadcast spawning coral species. PLoS ONE, 5(5). https://doi.org/10.1371/journal.pone.0010898
Vargas-Ángel, B., Peters, E. C., Kramarsky-Winter, E., Gilliam, D. S., & Dodge, R. E. (2007). Cellular reactions to sedimentation and temperature stress in the Caribbean coral Montastraea cavernosa. Journal of Invertebrate Pathology, 95(2). https://doi.org/10.1016/j.jip.2007.01.003
Veron, J. (2000) Corals of the World, Volume 1, Australian Institute of Marine Science, Townsville, Queensland, Australia.
Zilberberg, C., & Edmunds, P. J. (1999). Patterns of skeletal structure variability in clones of the reef coral Montastraea franksi. Bulletin of Marine Science, 64(2).
Zilberg, C., and Edmunds, P. (1999) Patterns of skeletal structure variability in clones of the reef coral Montastrea franksii. Bulletin of Marine Science, 64, 373-381
 Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Official websites use .gov
A .gov website belongs to an official government organization in the United States. Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.