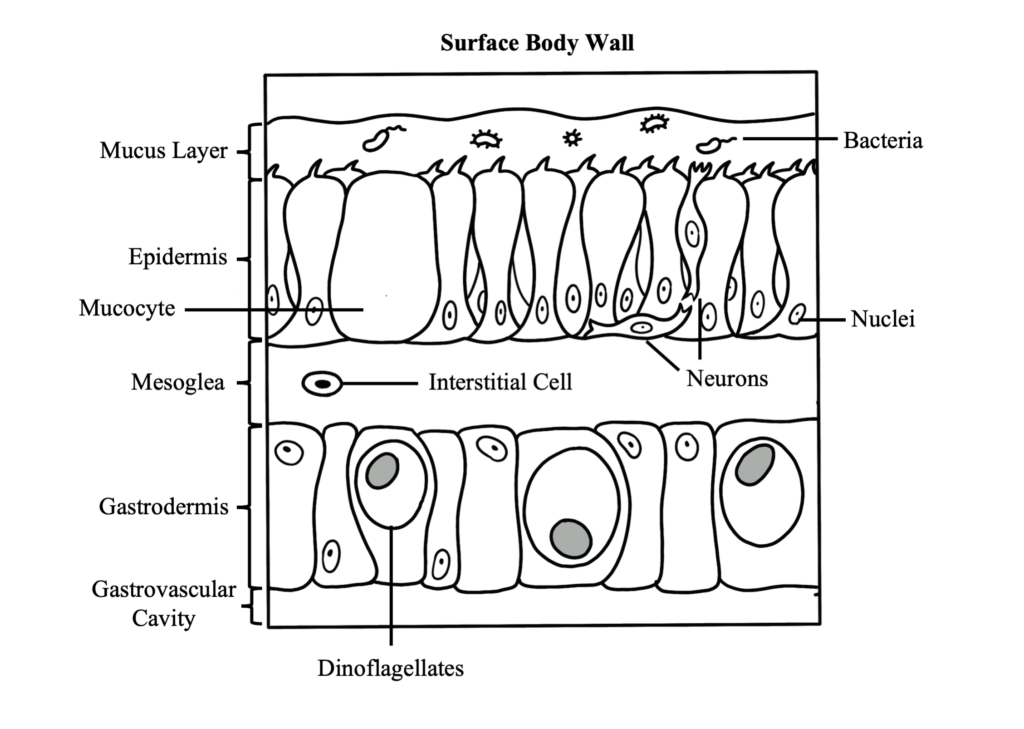
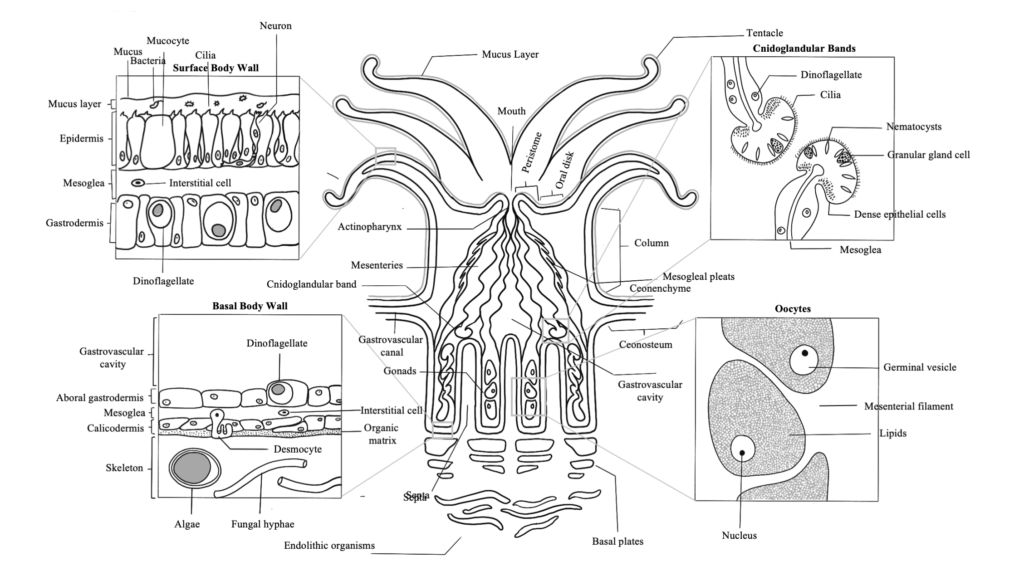
Coral Reproduction
Corals, like many sessile organisms that are unable to search for compatible partners, have adapted unique reproductive strategies that allow their eggs and sperm to mix and fertilize despite their immobility. In addition to the many factors that now threaten reefs throughout the world, such as climate change and pollution, there are many more challenges associated with the processes of reproduction and development that further threaten reef replenishment and persistence.
Asexual vs. Sexual Reproduction
Corals are able to reproduce asexually or sexually. Asexual reproduction occurs when one colony produces an additional colony that is genetically identical to itself without gamete involvement. Sexual reproduction occurs through the production of gametes (eggs and sperm) that are mixed during spawning, where eggs are fertilized and genetically unique embryos develop into larvae.
Asexual Reproduction
Asexual reproduction includes several strategies where tissues from one colony become separated and form a new colony:
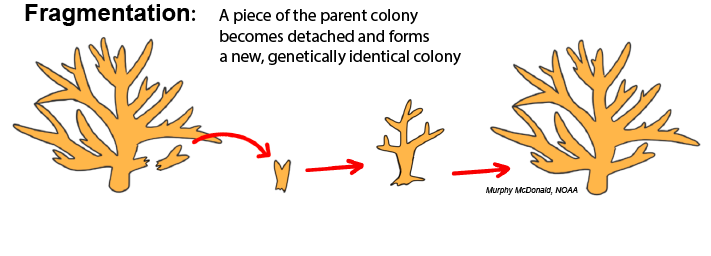
Fragmentation: Fragmentation occurs when a piece of tissue from a parent colony, either including skeleton or not, becomes dislodged from that colony, comes into contact with a suitable solid surface, and fuses with that substrate to begin growing a separate colony. This is common in branching and plating corals whose morphology creates thin and fragile extremities that are subject to breaking from physical impact. The principles of fragmentation are used as a technique for coral restoration programs, which “farm” propagules of parent colonies for replenishing dwindling reefs.
Polyp bailout: In polyp bailout, the tissue of an individual coral polyp lifts from the underlying skeleton and leaves the colony with aid from the cilia that line its outer surface. This form of tissue separation is unique from fragmentation in that it is a physiological response by the coral itself to dislodge tissue, rather than a passive process that results from physical trauma.
Parthenogenesis: Parthenogenesis is a unique and rare form of asexual reproduction where unfertilized eggs can develop into larvae without any contact with sperm. Parthenogenesis has been observed in Porites astreoides, Pocillopora damicornis, Pocillopora acuta, Tubastrea coccinea, Tubastrea diaphana, and Oulastrea crispata, though there are likely many more species that have the ability to employ this reproductive strategy that have yet to be studied or documented.
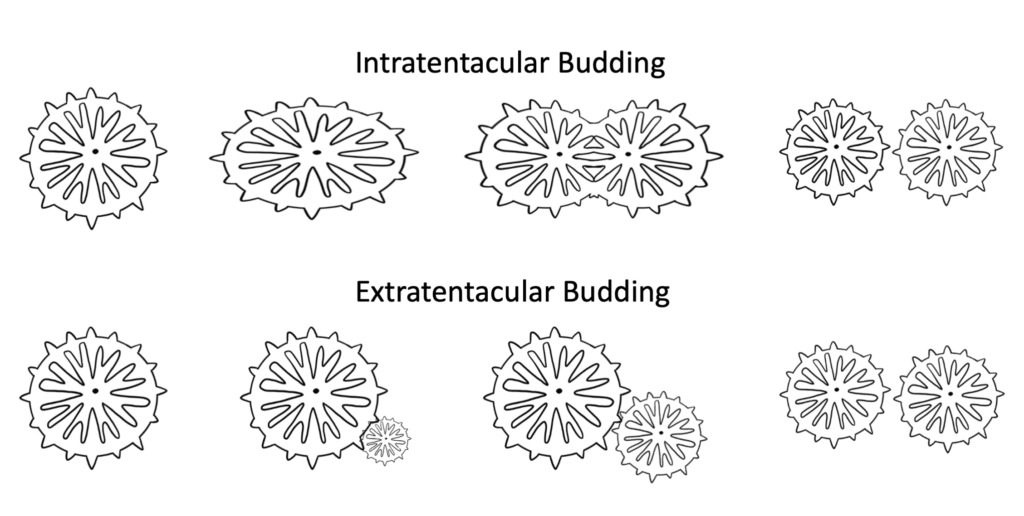
Budding: Budding occurs when a single coral polyp is formed either through the division of an existing polyp (intretentacular budding) or from the space between polyps (extratentacular budding). Polyps formed by this process remain intact and a part of the same colony, so this process is more often referred to as a means of growth than of reproduction.
Because asexual reproduction results in offspring that are genetically identical to the parent colony, those offspring should perform as well as their parent in a given habitat as long as their environment remains the same. However, if the environment experiences a disturbance, low genetic diversity within a population can be detrimental. Populations with low genetic diversity are vulnerable because of the low variability of resistance and susceptibility among individuals to different stressors, whether they be physical disturbances, such as storms, or biological threats, such as the introduction of new pathogens into the ecosystem. For this reason, a form of sexual reproduction is necessary for population longevity.
Sexual Reproduction
Sexual reproduction requires the fusion of eggs and sperm to form embryos. There are two reproductive modes used by corals to achieve this gamete mixing:
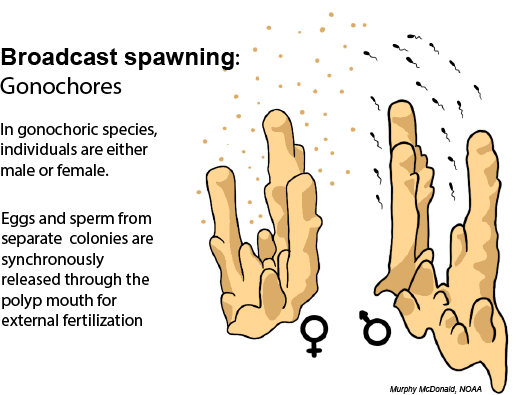
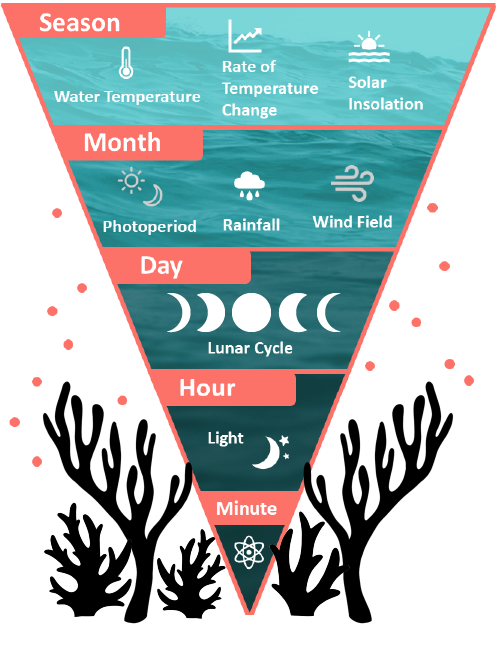
Broadcast spawning: Broadcast spawning species release their eggs and sperm into the water column (a process called “spawning”) where they are able to be fertilized and the resulting embryos develop into larvae externally. To ensure that the eggs and sperm from different individuals have a high chance of meeting, colonies in the same population commonly spawn at the same time in annual spawning events. This synchronized spawning window typically occurs at the same time every year, depending on the temperature and moon cycle. Read more about the environmental cues that corals rely on to synchronize their spawning below.
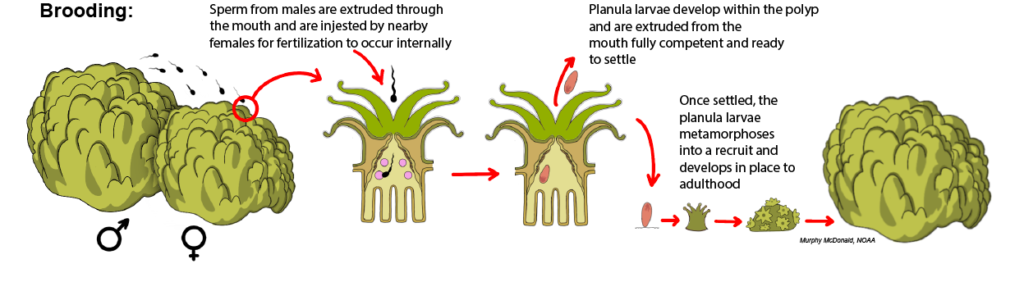
Brooding: In brooding species, fertilization and larval development occur internally within the polyps of the female colony. Nearby males release their sperm into the water column, which then comes into contact with the polyps of a female colony and is ingested through the polyp mouth for fertilization within the gastrovascular cavity. Because development occurs internally, the planula larvae that are released from the polyp mouth are typically larger than those produced by broadcast spawning species, which rely solely on the nutrients of the egg yolk for their development. Only about 15% of corals are reported to be brooding species.
Synchronized Spawning
To coordinate a synchronized spawning event with all of the nearby individuals in a given population, corals rely on a series of environmental and chemical cues to signal gametogenesis (gamete development within the mesoglea of the polyp tissues) and gamete release. This maximizes the chances that the gametes from one individual will mix with those from another, which is critical for sessile organisms such as corals that are unable to search for compatible partners through relocation.
Most species spawn only once a year, though some species, particularly brooders, are able to have multiple spawning events over consecutive months. Evidence suggests that the seasonal water temperature, the rate of temperature change between seasons, and the solar insolation all serve to signal the spawning season. On a smaller scale, the regional wind field, rainfall patterns, and photoperiod (night-to-day ratio) can finer tune the spawning season to the month. The exact day of spawning is signaled by the moon phase, with most broadcast spawning species reproducing around the full moon and most brooding species releasing larvae around the new moon. On the night of spawning, the precise time of gamete and larval release is thought to be signaled by nocturnal illumination, either total darkness after sunset or irradiance after moon rise. For this reason, “minutes after sunset”, or MAS, is often how the spawning time for different species is recorded. There is also evidence that chemical cues such as hormones play a role in signaling spawning in nearby corals, though the exact mechanisms of this process have not been determined.
Assisted Coral Reproduction
For many years, scientists have worked to utilize this predictable spawning pattern to study coral reproduction and to harness “assisted reproduction” as a reef restoration technique. Recently, breakthroughs in coral husbandry and aquaculture by scientists at the Horniman Museum have allowed scientists to synthetically recreate the environmental cues necessary to signal gametogenesis and spawning in an aquarium setting, revolutionizing the study of coral reproduction and greatly enhancing the potential for meaningful progress in reef restoration.
Larval Development, Dispersal, and Settlement
Corals have a life history with two distinct phases: the larval phase, where the coral is motile, and the sessile phase, where the coral is cemented to a substrate and unable to move on its own.
Once an embryo is formed after the fusion of compatible eggs and sperm, it undergoes a distinct series of phases as it develops into the characteristic “planula larvae” that cnidarians are known for.
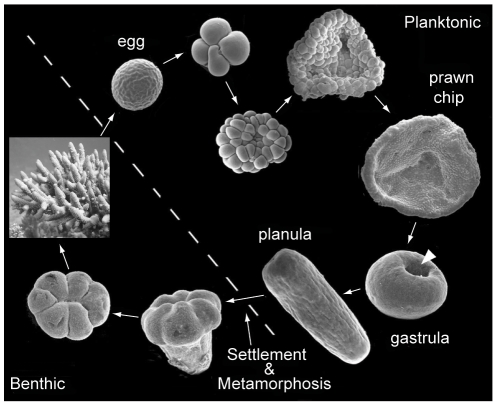
For broadcast spawning species, whose fertilization occurs externally, these stages of development occur in the water column, and the embryo attains its energy from the finite yolk provided by the egg. Once the larvae have developed cilia in the planula stage, they transition from pleuston (unable to control their own movement, at the mercy of prevailing currents) to neuston (able to control their own movement and begin directional swimming). For brooding species, development occurs internally within the female adult, and the larvae are released in the planula stage and competent for swimming. Because these brooded larvae are able to attain nutrients from the parent during development, they are generally larger than the planula of broadcast spawning species.
Once the planula larvae are “competent” to settle, i.e., they are capable to directional swimming, a variety of cues guide them to suitable reef substrate for permanent settlement and growth into adulthood. The most potent cue is a chemical signal released by certain species of crustose coralline algae, but planula have also been shown to respond to signals released by adults of the same species, certain biofilms, and light.
When a larva has found a suitable substrate, it settles onto it and begins the irreversible processes of metamorphosis into its juvenile phase, which is segmented and includes a mouth. The arrangement of the skeleton in this first polyp differs by genus and is a useful tool for identification of juveniles. From this point, species differ in their growth rates in terms of budding additional polyps, developing tentacles, and acquiring the algal symbionts that they require for survival.
The juvenile stage of corals is the most vulnerable after its planktonic larval stage, and presents a substantial bottleneck for successful recruitment of new individuals into a reef population. Competition with other benthic species, particularly with macroalgae that is fast growing and can smother a small coral juvenile, as well as predation from corallivorous snails and fish present some of the many biological threats that can prevent successful recruitment, as well as abiotic pressures such as smothering by sedimentation and pollution. It is for this reason that many reef restoration practitioners have targeted this life stage to focus protection measures and interference that will increase survival into adulthood.
Learn More about the Essentials of Coral Biology:
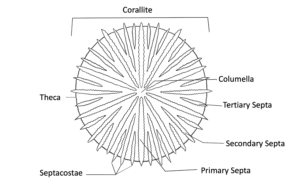
Coral Skeleton
Learn about all of the components of the coral skeleton and the different morphologies that it can display
Publications Relevant to Coral Reproduction:
Alamaru, A., Yam, R., Shemesh, A., & Loya, Y. (2009). Trophic biology of stylophora pistillata larvae: Evidence from stable isotope analysis. Marine Ecology Progress Series, 383. https://doi.org/10.3354/meps07958
Arai, I., Kato, M., Heyward, A., Ikeda, Y., Iizuka, T., & Maruyama, T. (1993). Lipid composition of positively buoyant eggs of reef building corals. Coral Reefs, 12(2). https://doi.org/10.1007/BF00302104
Armoza-Zvuloni, R., Segal, R., Kramarsky-Winter, E., & Loya, Y. (2011). Repeated bleaching events may result in high tolerance and notable gametogenesis in stony corals: Oculina patagonica as a model. Marine Ecology Progress Series, 426. https://doi.org/10.3354/meps09018
Ayre, D. J., & Resing, J. M. (1986). Sexual and asexual production of planulae in reef corals. Marine Biology, 90(2). https://doi.org/10.1007/BF00569126
Babcock, R. C. (1991). Comparative demography of three species of scleractinian corals using age- and size-dependent classifications. In Ecological Monographs (Vol. 61, Issue 3). https://doi.org/10.2307/2937107
Babcock, R. C., & Heyward, A. J. (1986). Larval development of certain gamete-spawning scleractinian corals. Coral Reefs, 5(3). https://doi.org/10.1007/BF00298178
Babcock, R. C., Bull, G. D., Harrison, P. L., Heyward, A. J., Oliver, J. K., Wallace, C. C., & Willis, B. L. (1986). Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Marine Biology, 90(3). https://doi.org/10.1007/BF00428562
Babcock, R. C., Wills, B. L., & Simpson, C. J. (1994). Mass spawning of corals on a high latitude coral reef. Coral Reefs, 13(3). https://doi.org/10.1007/BF00301193
Baird, A. H., Guest, J. R., & Willis, B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annual Review of Ecology, Evolution, and Systematics, 40. https://doi.org/10.1146/annurev.ecolsys.110308.120220
Bassim, K. M., Sammarco, P. W., & Snell, T. L. (2002). Effects of temperature on success of (self and non-self) fertilization and embryogenesis in Diploria strigosa (Cnidaria, Scleractinia). Marine Biology, 140(3). https://doi.org/10.1007/s00227-001-0722-4
Brady, A. K., Hilton, J. D., & Vize, P. D. (2009). Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. In Coral Reefs (Vol. 28, Issue 3). https://doi.org/10.1007/s00338-009-0498-4
Brazeau, D. A., Gleason, D. F., & Morgan, M. E. (1998). Self-fertilization in brooding hermaphroditic Caribbean corals: Evidence from molecular markers. Journal of Experimental Marine Biology and Ecology, 231(2). https://doi.org/10.1016/S0022-0981(98)00097-5
CHORNESKY, E. A., & PETERS, E. C. (1987). SEXUAL REPRODUCTION AND COLONY GROWTH IN THE SCLERACTINIAN CORAL PORITES ASTREOIDES . The Biological Bulletin, 172(2). https://doi.org/10.2307/1541790
Esquivel, I. F. (1986). Short term copper bioassay on the planula of the reef coral Pocillopora damicornis. Coral Reef Population Biology. Jokiel, P.L.;Richmond, R.H.;Rogers, R.a. Eds.
Fadlallah, Y. H. (1983). Sexual reproduction, development and larval biology in scleractinian corals – A review. In Coral Reefs (Vol. 2, Issue 3). https://doi.org/10.1007/BF00336720
Fadlallah, Y. H., & Pearse, J. S. (1982). Sexual reproduction in solitary corals: Overlapping oogenic and brooding cycles, and benthic planulas in Balanophyllia elegans. Marine Biology, 71(3). https://doi.org/10.1007/BF00397039
Fukami, H., Omori, M., Shimoike, K., Hayashibara, T., & Hatta, M. (2003). Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Marine Biology, 142(4). https://doi.org/10.1007/s00227-002-1001-8
Glynn, P. W., Gassman, N. J., Eakin, C. M., Cortes, J., Smith, D. B., & Guzman, H. M. (1991). Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador) – I. Pocilloporidae. Marine Biology, 109(3). https://doi.org/10.1007/BF01313501
Goffredo, S., Gasparini, G., Marconi, G., Putignano, M. T., Pazzini, C., & Zaccanti, F. (2010). Gonochorism and planula brooding in the mediterranean endemic orange coral Astroides calycularis (Scleractinia: Dendrophylliidae). Morphological aspects of gametogenesis and ontogenesis. Marine Biology Research, 6(5). https://doi.org/10.1080/17451000903428488
Guest, J. R., Baird, A. H., Goh, B. P. L., & Chou, L. M. (2012). Sexual systems in scleractinian corals: An unusual pattern in the reef-building species Diploastrea heliopora. Coral Reefs, 31(3). https://doi.org/10.1007/s00338-012-0881-4
Hagman, D. K., Gittings, S. R., & Deslarzes, K. J. P. (1998). Timing, species participation, and environmental factors influencing annual mass spawning at the Flower Garden Banks (northwest Gulf of Mexico). Gulf of Mexico Science, 16(2). https://doi.org/10.18785/goms.1602.06
Hall, V. R., & Hughes, T. P. (1996). Reproductive strategies of modular organisms: Comparative studies of reef-building corals. Ecology, 77(3). https://doi.org/10.2307/2265514
Hanafy, M. H., Aamer, M. A., Habib, M., Rouphael, A. B., & Baird, A. H. (2010). Synchronous reproduction of corals in the Red Sea. Coral Reefs, 29(1). https://doi.org/10.1007/s00338-009-0552-2
Harii, S., Kayanne, H., Takigawa, H., Hayashibara, T., & Yamamoto, M. (2002). Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulea and Pocillopora damicornis. Marine Biology, 141(1). https://doi.org/10.1007/s00227-002-0812-y
Harii, S., Nadaoka, K., Yamamoto, M., & Iwao, K. (2007). Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Marine Ecology Progress Series, 346. https://doi.org/10.3354/meps07114
Harii, S., Yamamoto, M., & Hoegh-Guldberg, O. (2010). The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Marine Biology, 157(6). https://doi.org/10.1007/s00227-010-1401-0
Harriott, V. J. (1983). Reproductive ecology and population dynamics in a scleractinian coral community. Department of Marine Biology, PhD.
Harrison, P. L. (2011). Sexual reproduction of scleractinian corals. In Coral Reefs: An Ecosystem in Transition. https://doi.org/10.1007/978-94-007-0114-4_6
Harrison, R. L., Babcock, R. C., Bull, G. D., Oliver, J. K., Wallace, C. C., & Willis, B. L. (1984). Mass spawning in tropical reef corals. Science, 223(4641). https://doi.org/10.1126/science.223.4641.1186
Heyward, A. J., Yamazato, K., Yeemin, T., & Minei, M. (1987). Sexual reproduction of corals in Okinawa. Galaxea, 6.
Highsmith, R. (1982). Reproduction by Fragmentation in Corals. Marine Ecology Progress Series, 7. https://doi.org/10.3354/meps007207
Hirose, M., & Hidaka, M. (2006). Early development of zooxanthella-containing eggs of the corals Porites cylindrica and Montipora digitata: The endodermal localization of zooxanthellae. Zoological Science, 23(10). https://doi.org/10.2108/zsj.23.873
Hirose, M., Kinzie, R., & Hidaka, M. (2001). Timing and process of entry of zooxanthellae into oocytes of hermatypic corals. Coral Reefs, 20(3). https://doi.org/10.1007/s003380100171
Hirose, M., Yamamoto, H., & Nonaka, M. (2008). Metamorphosis and acquisition of symbiotic algae in planula larvae and primary polyps of Acropora spp. Coral Reefs, 27(2). https://doi.org/10.1007/s00338-007-0330-y
Humphrey, C., Weber, M., Lott, C., Cooper, T., & Fabricius, K. (2008). Effects of suspended sediments, dissolved inorganic nutrients and salinity on fertilisation and embryo development in the coral Acropora millepora (Ehrenberg, 1834). Coral Reefs, 27(4). https://doi.org/10.1007/s00338-008-0408-1
Jokiel, P. L., & Guinther, E. B. (1978). Effects of temperature on reproduction in the hermatypic coral Pocillopora damicornis. Bulletin of Marine Science, 28(4).
Jokiel, P. L., Ito, R. Y., & Liu, P. M. (1985). Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Marine Biology, 88(2). https://doi.org/10.1007/BF00397164
Kai, S., & Sakai, K. (2008). Effect of colony size and age on resource allocation between growth and reproduction in the corals Goniastrea aspera and Favites chinensis. Marine Ecology Progress Series, 354. https://doi.org/10.3354/meps07216
Kojis, B. L., & Quinn, N. J. (1981). Aspects of sexual reproduction and larval development in the shallow water Hermatypic Coral, Goniastrea australensis (Edwards and Haime, 1857). Bulletin of Marine Science, 31.
Kojis, B., & Quinn, N. (1982). Reproductive Ecology of Two Faviid Corals (Coelenterata: Scleractinia). Marine Ecology Progress Series, 8. https://doi.org/10.3354/meps008251
Kozłowski, J., & Wiegert, R. G. (1986). Optimal allocation of energy to growth and reproduction. Theoretical Population Biology, 29(1). https://doi.org/10.1016/0040-5809(86)90003-1
Kramarsky-Winter, E., & Loya, Y. (1998). Reproductive strategies of two fungiid corals from the northern Red Sea: Environmental constraints? Marine Ecology Progress Series, 174. https://doi.org/10.3354/meps174175
Krupp, D. A. (1983). Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs, 2(3). https://doi.org/10.1007/BF00336722
 Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Official websites use .gov
A .gov website belongs to an official government organization in the United States. Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.